How plants bend towards
light?
Prof. R. P. Sharma
School of Life Sciences
University of Hyderabad
Hyderabad-500046
Introduction:
The life on our planet is sustained
by continuous input of energy in biosphere in the form of the sunlight,
which is harvested by plants, via a process called photosynthesis. Not
only the plants use sunlight as a source of energy, to drive the process
of photosynthesis, they have also evolved several specialized mechanisms,
where they use the sunlight as the source of the information. Plants have
developed mechanisms to sense the impending seasonal changes in the climate,
to detect the shading and competition from the neighboring plants, to follow
the path of the Sun etc. The bending of the plants towards the source of
light, such as orientation of an indoor plant towards window of the room,
is one of such well known phenomena. Each of these processes uses the light
as a source of information and integrates this information with growth
and development of plants. The processes involved in the detection of the
light, transduction of light information signal to a chemical signal, to
final execution of the photoresponse, consists of multiple steps and involves
a coordinated process between different tissues and organs. The detailed
steps involved in these processes are still not fully deciphered and are
under intensive investigation. In the present talk I have summarized the
current knowledge about this phenomena.
Historical Perspective
Though the orientation of plants
towards the source of the sunlight is a well known phenomenon even to a
layman, the first scientist to critically examine this process was none
other than Charles Darwin. During his long voyage on the famous ship Beagle,
Darwin kept the birds captured during his journey. He fed these birds with
seedlings raised out of canary (Phalaris sp) grass, which he grew in small
containers in his cabin in the ship. Being a keen naturalist he observed
that the seedlings grown in his cabin were oriented towards the window,
which was the only source of light in the dark cabin. He followed these
observations with the experiments, which he eventually along with his son
as a co-author published as a monograph namely "The power of the movement
in the plants". Darwin showed that in the absence of light, a germinating
seed's coleoptile grew vertically. The coleoptile of the same seedlings
on exposure to unidirectional light bent towards the light source. However,
if the coleoptile tip was removed or covered by an opaque cap, coleoptiles
did not bend towards light. Whereas, the bending toward the light source
occurred when the coleoptiletip was covered by a transparent cap or the
region where bending occurred was covered by an opaque material.
These experiments of Darwin laid the foundation for the modern plant hormonal
physiology, which eventually lead to discovery that the coleoptile tip
was the site of auxin synthesis. However, we first like to learn how would
the coleoptile tip or a plant sense that the light is unidirectional in
nature?
Plants see the light using specialized
photoreceptors
Plants can see the light, the very
same way, which we do, by using specific macromolecules called as photoreceptors
that can 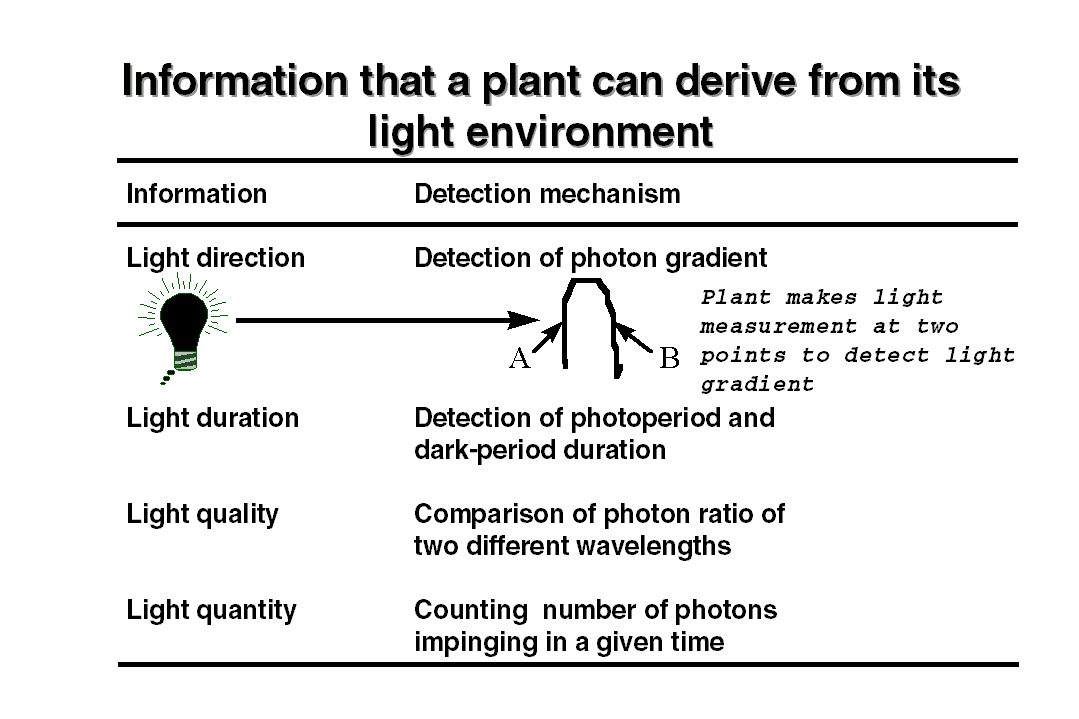 detect
the light. In case of mammals rhodospin is the visual pigment of the eye.
Plants too have similar type of the photoreceptor molecules to gather information
about its light environment. Plants obtain information about its light
environment by several ways using one of the properties of the light. The
way plants elicit this information is illustrated in the table. To detect
an unidirectional light the plants have to make a two point measurement
to detect a light gradient within the plant body. In the natural environment
plants use different photoreceptors to collect the information about light
gradient, duration, photoperiod etc. For detecting each type of information,
plants have evolved multiple photoreceptors to perform these tasks. There
are enough physiological evidences for existence of different
detect
the light. In case of mammals rhodospin is the visual pigment of the eye.
Plants too have similar type of the photoreceptor molecules to gather information
about its light environment. Plants obtain information about its light
environment by several ways using one of the properties of the light. The
way plants elicit this information is illustrated in the table. To detect
an unidirectional light the plants have to make a two point measurement
to detect a light gradient within the plant body. In the natural environment
plants use different photoreceptors to collect the information about light
gradient, duration, photoperiod etc. For detecting each type of information,
plants have evolved multiple photoreceptors to perform these tasks. There
are enough physiological evidences for existence of different 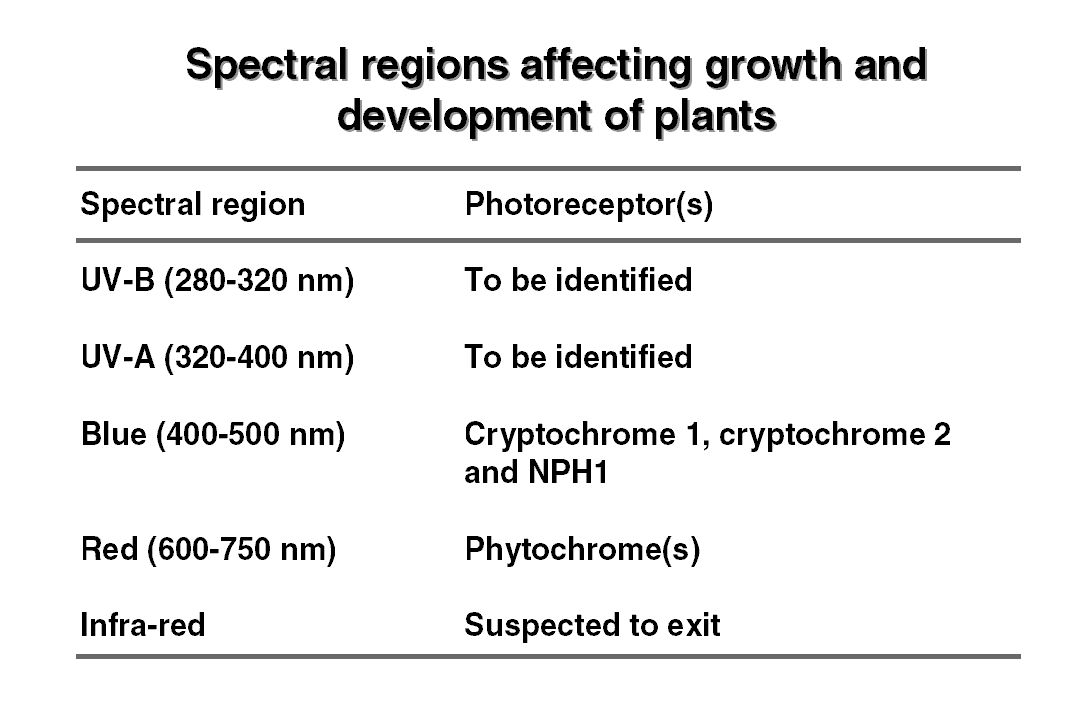 photoreceptors,
however for most photoreceptors their biochemical nature is yet to
be identified. The known and yet to be identified photoreceptors of plants
are listed in table.
photoreceptors,
however for most photoreceptors their biochemical nature is yet to
be identified. The known and yet to be identified photoreceptors of plants
are listed in table.
How does one identify the photoreceptor?
The task of a photobiologist, once
he identifies the photoresponse, is to identify the photoreceptor causing
the response. Most often it is a job similar to finding a needle in the
haystack, however the photobiologists are added in this task by a technique
called action spectroscopy. The action spectra of photoresponses are derived
by a careful study of the photoresponse at  different
wavelengths. First, the given plant material is exposed to different doses
of light at a given wavelength and a dose response curve for the photoresponse
is plotted. Similar dose responses curves are then obtained for the other
wavelengths too, by increasing wavelength interval by 10 or 20 nm. Second,
using these dose response curves, the number of photons required to elicit
a fixed degree of the photoresponse at different wavelengths is determined.
It is naturally expected the least amount of quanta would be required for
eliciting the photoresponse at the most effective wavelength. The reciprocal
of quanta required to elicit the response are then plotted against the
wavelength and called as action spectra. The action spectra of a given
photoresponse are then compared with the absorption spectra of known photochromic
molecules. It is implicit in these comparative studies that the action
spectra so obtained closely represent the absorption spectra of photoreceptor
causing the photoresponse. In past the action spectroscopy has been successfully
used to identify and isolate the plant photoreceptor phytochrome, which
plays a major role in photoperiodic regulation of flowering in higher plants.
different
wavelengths. First, the given plant material is exposed to different doses
of light at a given wavelength and a dose response curve for the photoresponse
is plotted. Similar dose responses curves are then obtained for the other
wavelengths too, by increasing wavelength interval by 10 or 20 nm. Second,
using these dose response curves, the number of photons required to elicit
a fixed degree of the photoresponse at different wavelengths is determined.
It is naturally expected the least amount of quanta would be required for
eliciting the photoresponse at the most effective wavelength. The reciprocal
of quanta required to elicit the response are then plotted against the
wavelength and called as action spectra. The action spectra of a given
photoresponse are then compared with the absorption spectra of known photochromic
molecules. It is implicit in these comparative studies that the action
spectra so obtained closely represent the absorption spectra of photoreceptor
causing the photoresponse. In past the action spectroscopy has been successfully
used to identify and isolate the plant photoreceptor phytochrome, which
plays a major role in photoperiodic regulation of flowering in higher plants.
Action spectroscopy revealed that
plant bending is caused by blue light
The action spectroscopy revealed
that the effective spectral region triggering phototropism is in between
350-500 nm i.e. the blue region of the spectrum. The action spectra of
oat coleoptile phototropism showed two prominent peaks at 475 and 450 nm
with a shoulder at 420 nm. Interestingly the action spectrum for phototropic
curvature in sporangiophore of the fungus Phycomyces also shows features
similar to oat coleoptile with peak at 460 nm. It is of interest to note
that the Nobel Laureate Max. Delbrück, after his pioneering work on
bacteriophage genetics, for which he got the Nobel award, shifted his research
area to study of phototropism in Phycomyces. These similarities highlight
that photoreceptor mediating phototropism in higher plants and fungus may
share few common elements.
Molecular nature of the blue light
photoreceptor's chromophore
The similarity between the action
spectra and absorption spectra of known biological pigments has been used
to predict the likely molecular nature of the photoreceptor, or at least
the nature of chromophore associated with the photoreceptor. Based on 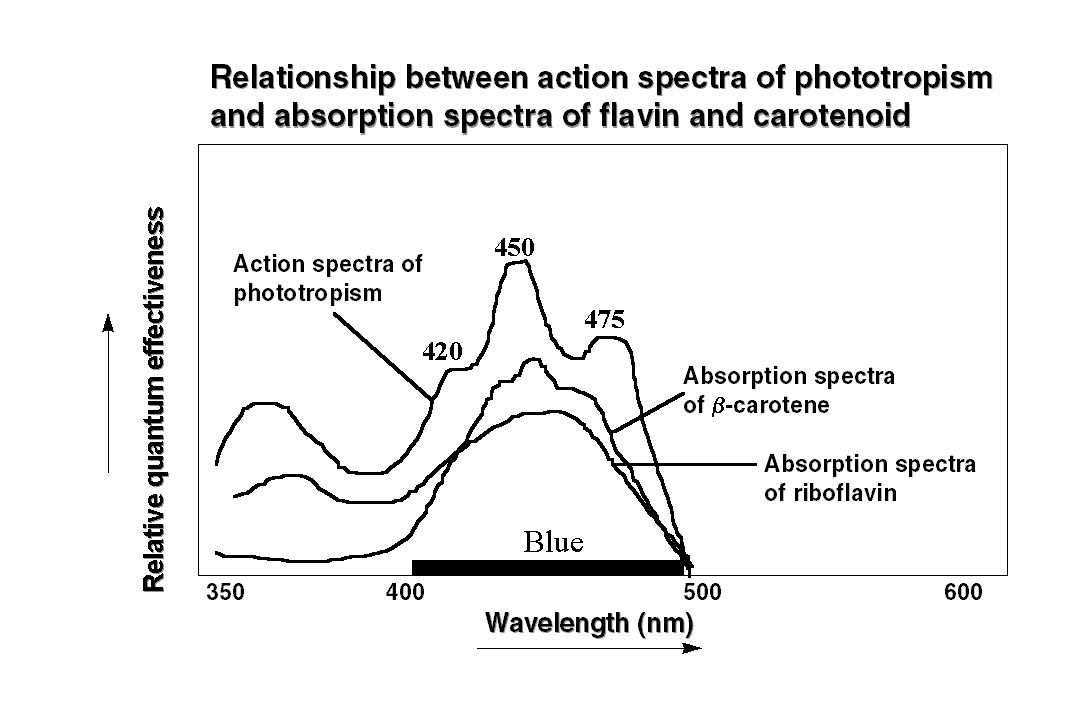 the
similarity between action spectra of phototropism and absorption spectra,
it has been predicted that a carotenoid, or a flavin, or a pterin act as
putative chromophore for the photoreceptor. Arguments and evidences for
and against each of these molecules have been advanced in the past. In
recent years most people have favored flavin and pterin as potential candidates
for the chromophore, however, still few people support carotenoid as chromophore
molecule. A great deal of the controversy regarding the molecular nature
of the chromophore has been generated due to lack of identification of
the photoreceptor molecules per se. However, in recent years the remarkable
progress has been made for identification for at least few likely photoreceptor
candidates, which is described below.
the
similarity between action spectra of phototropism and absorption spectra,
it has been predicted that a carotenoid, or a flavin, or a pterin act as
putative chromophore for the photoreceptor. Arguments and evidences for
and against each of these molecules have been advanced in the past. In
recent years most people have favored flavin and pterin as potential candidates
for the chromophore, however, still few people support carotenoid as chromophore
molecule. A great deal of the controversy regarding the molecular nature
of the chromophore has been generated due to lack of identification of
the photoreceptor molecules per se. However, in recent years the remarkable
progress has been made for identification for at least few likely photoreceptor
candidates, which is described below.
Dose response curve of phototropism
has two peaks
Normally the dose responses
curves for most photoresponses are linear in shape till it reaches a plateau.
However the dose response curves for the phototropism in higher plants
have a very distinct shape, consisting of three zones. The initial rising
part of the curve and the first peak is called as first-positive curvature,
the subsequent decline to a minimum is called as first-negative curvature,
and the second rising part is called as the second-positive curvature.
This unusual shape of the dose response curve 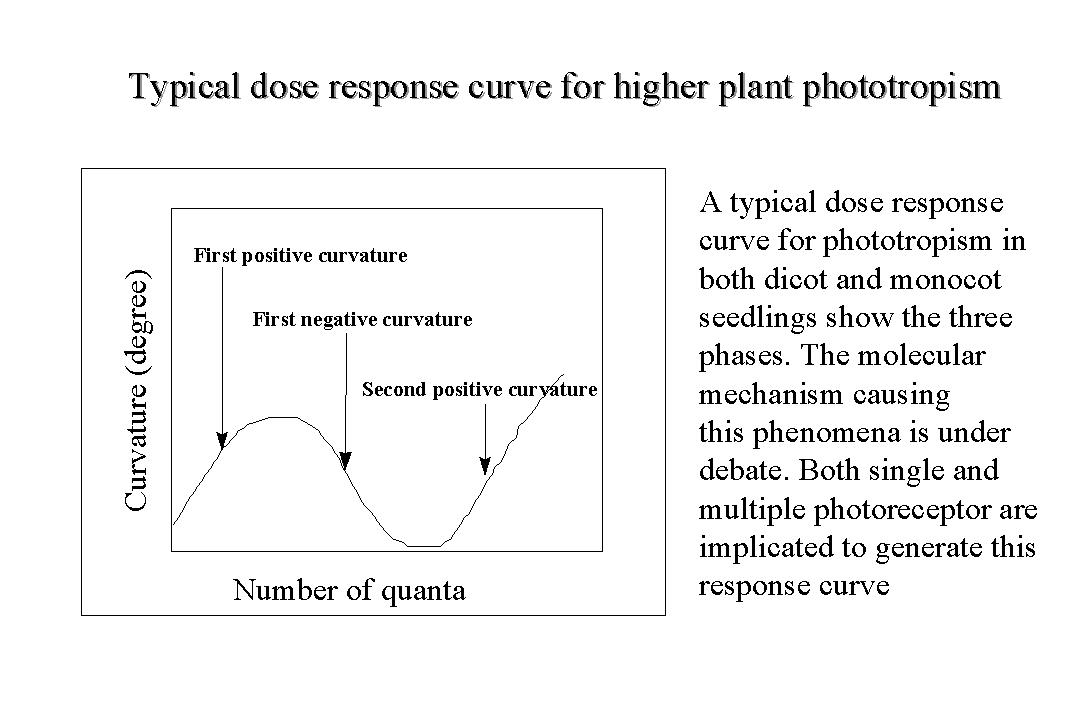 for
the phototropic curvature has added to the complexities of the identification
of the photoreceptors. The views for and against the multiple photoreceptors
have been advanced on the basis of the physiological consideration. The
recent results on identification of the photoreceptors have not still clearly
resolved the phenomena. The evidence is still incomplete to say firmly
if one or more than one photoreceptor sense phototropic blue light.
for
the phototropic curvature has added to the complexities of the identification
of the photoreceptors. The views for and against the multiple photoreceptors
have been advanced on the basis of the physiological consideration. The
recent results on identification of the photoreceptors have not still clearly
resolved the phenomena. The evidence is still incomplete to say firmly
if one or more than one photoreceptor sense phototropic blue light.
One of the earliest responses
in the phototropism is phosphorylation of a membrane bound protein
The etiolated seedlings of
higher plants are hypersensitive to blue light, even a brief exposure of
one or two second of weak blue light is sufficient to elicit phototropic
curvature. The work carried out by the Winslow Briggs group revealed that
exposure to blue light for such a short duration causes phosphorylation
of a 120 kD protein likely associated with the membranes. Both in vivo
irradiation of etiolated plants or in vitro irradiation of the homogenates
could elicit the protein phosphorylation. Moreover, the phosphorylation
was observed in most of the higher plants examined including both dicots
and monocots. The physiological studies conducted showed a close correlation
between the phototropic responses and blue light mediated phosphorylation.
It was speculated that this phosphorylation might represent one of the
initial events in the phototropic signal transduction pathway.
Mutants have aided the identification
of photoreceptors
The physiological studies during
the past century though aided to characterization of the phototropic response,
the molecular identity of the photoreceptor could not be ascertained. However
during past decade a significant progress was made to identify the photoreceptors
by isolating mutants from Arabidopsis, also known as "Botanical Drosophila".
Jiten Khurana while in Ken Poff’s lab was first to actively screen Arabidopsis
for the phototropic mutants. He isolated several mutants defective in phototropism,
which were eventually found to be defective in the photoreceptor and also
in the components of the signal transduction chain. A proof that the blue
light mediated phosphorylation was indeed the part of phototropic response
was obtained using those mutants. The mutant JK224 which was defective
in the phototropism was also defective in phosphorylation. Winslow Briggs
supplemented to the studied of Khurana by isolating few more mutants and
rechristened JK mutants as nph (non-phototropic hypocotyl)
mutants in Arabidopsis. These mutants were found to be defective in the
signal transduction and photoreceptor per se. The molecular analysis
of these mutants particularly of nph1 mutant lead to the identification
and isolation of first putative photoreceptor for the phototropism.
NPH1 gene encodes a kinase and
has a flavin binding site
The NPH1 gene was cloned using
the technique of Amplified Fragment Length Polymorphism in 1997. The perusal
of the NPH1 gene sequence and its comparison with other gene sequences
in the data bases showed that it encodes a novel protein with significant
homology with proteins with LOV domain found in bacteria and higher organism.
The gene sequence also indicated that the protein encodes a serine-threonine
protein kinase. The protein has a putative flavin-binding site too, confirming
for the first time that flavin rather than carotenoid act as chromophore
for phototropism. The confirmation that NPH1 protein is the photoreceptor
for the phototropism rather than an intermediate in the signal pathway
came for its expression studies. The NPH1 protein can be expressed in the
insect's cells and does undergo autophosphorylation on exposure to the
blue light. Recombinant NPH1 binds FMN noncovalently and its fluorescence
excitation spectrum is similar to action spectrum of the phototropism.
Since mutant defective in the NPH1 protein lacks phototropic responses,
it is believed that NPH1 is the photoreceptor eliciting phototropism. The
NPH1 protein has been renamed phototropin, to indicate
its biochemical function. Studies on structure and function of phototropin
are currently being done by Briggs laboratory to understand how this
protein mediates phototropic signaling.
Single/multiple photoreceptors
for phototropic response
The unique dose response curve
of the phototropic responses has been interpreted as a result of the operation
of two independent photoreceptors. The isolation of nph1 mutants indicated
that one single photoreceptor may be sufficient to trigger phototropism
in plants. However blue light also mediates other responses in the Arabidopsis
seedlings such as inhibition of hypocotyl elongation and promotion of cotyledon
expansion using two other photoreceptors namely cryptochrome 1 and cryptochrome
2 respectively. Similarly to NPH1 the cryptochrome 1 and cryptochrome 2
have also been cloned from Arabidopsis, and found to have strong homology
with microbial DNA photolyases. The cryptochromes are dual photochromic
molecules having two chromophore binding sites one for a flavin and another
for pterin. The mutants deficient in either cryptochrome 1 or cryptochrome
2 are normal in their phototropic behavior. However, a double mutant of
cry1/cry2 lacks the first-positive curvature of phototropism. Since the
double mutant shows normal blue light mediated phosphorylation of 120 kD
protein, it has been assumed that NPH1 is present and functions normally
in these mutants. It has been proposed that the first-positive curvature
is mediated by cooperative action of cryptochrome 1 and cryptochrome 2
genes, whereas NPH1 may be a component of the signal transduction chain
acting down stream of these photoreceptors. Since the above double mutants
retains normal second-positive curvature, it can be assumed that either
NPH1 or another yet unknown photoreceptor triggers this reaction. It is
expected that in coming years the detailed analysis of mutants would clarify
the relative roles of these photoreceptors in phototropism.
Additional photoreceptors may
be involved in phototropism
As outlined above, the current
knowledge obtained from studies on Arabidopsis, indicates that there are
at least three putative candidates for the blue light photoreceptor mediating
phototropism namely, NPH1, cryptochrome 1 and cryptochrome 2. It is likely
that there may additional photoreceptors in the plants, which may mediate
this response. The action spectra of phototropism have indicated
that the wavelength higher than 500 nm are ineffective in triggering phototropic
reaction. One of the major photoreceptor which sense the wavelength higher
than 500 nm is phytochrome which detects red/far-red regions of the spectrum.
It has been shown that the in blue light mediated responses phytochrome
plays only a modulatory role. The etiolated seedlings if exposed to a brief
pulse of red light about 1-2 hour prior to blue light exposure shows the
increase in the amplitude of the first-positive curvature compared to control
seedlings. However red light is ineffective in causing phototropic curvature.
An evidence for a major role of phytochrome in phototropic responses has
come from analysis of phototropism in phytochrome deficient mutants. These
studies highlighted that in Arabidopsis both phytochrome A and B are required
for manifestation of the phototropic responses. The double mutants of phytochrome
A and B respond very sluggishly to blue light and show extremely delayed
curvatures in Arabidopsis.
Differential growth is needed
for the expression of the response
Darwin identified tip as the
site of perception for phototropism and subsequently Went showed that tip
actively secreted plant hormone auxin. These two observation were then
rationalized to explain the phototropic curvature of plants by formulating
classical Went and Cholodny hypothesis. This hypothesis stated that the
differential growth during phototropism occurred due to redistribution
of auxin. Light caused a grater accumulation of auxin on the shaded side
resulting in the faster elongation growth on that side, compared to illuminated
side leading to curvature of the seedlings. There has been extensive debate
for and against his hypothesis. Some support for this hypothesis has recently
come from analysis of nph4 mutant of Arabidopsis, which is a signal
transduction mutant. This mutant shows defect both in phototropism and
geotropism and therefore a signal chain mutant. It also highlights that
the phototropism and geotropism may share the components of signal chain,
a prediction of Cholodny and Went hypothesis. The above mutant is defective
in auxin metabolism and show that auxin is needed for displaying normal
phototropic curvature in plants.
Conclusion
The research on plant phototropism
has finally seen the light at the end of the tunnel. The combination of
studies using genetics, molecular biology and physiology of plants and
the newly generated mutants of plant development would greatly add to our
understanding of these phenomena. It is expected that more elements in
the perception of light and signal transmission and execution of the response
would be identified. At the end I would like to mention that most research
on phototropism has been carried out using etiolated seedlings and we have
only little information on phototropism of green plants. It is expected
that in future further studies would provide us with a comprehensive picture
of the process of phototropism in plants.
References
Ahmad M, Jarillo JA, Smirnova
O, Cashmore AR (1998) Cryptochrome blue-light photoreceptors of Arabidopsis
implicated in phototropism. Nature 392:720-723
Christi JM, Reymond P, Powell
GK, Bernasconi P, Raibekas AA, Liscum E, and Briggs WR (1998) Arabidopsis
NPH1 has properties of a blue-light photoreceptor for phototropism. Science
282: 1698-1701
Huala E, Oeller PW, Liscum E,
Han E-S, Larsen E, and Briggs WR (1997) Arabidopsis NPH1: A protein
kinase with a putative redox-sensing domain. Science 278: 2120-2123
Liscum E and Briggs WR (1995)
Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic
stimuli. Plant Cell 7: 473-485
Stowe-Evans EL, Harper RM, Motchoulski
AV, and Liscum E (1998) NPH4, a conditional modulator of auxin-dependent
differential growth responses in Arabidopsis. Plant Physiol 118:
1265-1275
 detect
the light. In case of mammals rhodospin is the visual pigment of the eye.
Plants too have similar type of the photoreceptor molecules to gather information
about its light environment. Plants obtain information about its light
environment by several ways using one of the properties of the light. The
way plants elicit this information is illustrated in the table. To detect
an unidirectional light the plants have to make a two point measurement
to detect a light gradient within the plant body. In the natural environment
plants use different photoreceptors to collect the information about light
gradient, duration, photoperiod etc. For detecting each type of information,
plants have evolved multiple photoreceptors to perform these tasks. There
are enough physiological evidences for existence of different
detect
the light. In case of mammals rhodospin is the visual pigment of the eye.
Plants too have similar type of the photoreceptor molecules to gather information
about its light environment. Plants obtain information about its light
environment by several ways using one of the properties of the light. The
way plants elicit this information is illustrated in the table. To detect
an unidirectional light the plants have to make a two point measurement
to detect a light gradient within the plant body. In the natural environment
plants use different photoreceptors to collect the information about light
gradient, duration, photoperiod etc. For detecting each type of information,
plants have evolved multiple photoreceptors to perform these tasks. There
are enough physiological evidences for existence of different  photoreceptors,
however for most photoreceptors their biochemical nature is yet to
be identified. The known and yet to be identified photoreceptors of plants
are listed in table.
photoreceptors,
however for most photoreceptors their biochemical nature is yet to
be identified. The known and yet to be identified photoreceptors of plants
are listed in table.
 different
wavelengths. First, the given plant material is exposed to different doses
of light at a given wavelength and a dose response curve for the photoresponse
is plotted. Similar dose responses curves are then obtained for the other
wavelengths too, by increasing wavelength interval by 10 or 20 nm. Second,
using these dose response curves, the number of photons required to elicit
a fixed degree of the photoresponse at different wavelengths is determined.
It is naturally expected the least amount of quanta would be required for
eliciting the photoresponse at the most effective wavelength. The reciprocal
of quanta required to elicit the response are then plotted against the
wavelength and called as action spectra. The action spectra of a given
photoresponse are then compared with the absorption spectra of known photochromic
molecules. It is implicit in these comparative studies that the action
spectra so obtained closely represent the absorption spectra of photoreceptor
causing the photoresponse. In past the action spectroscopy has been successfully
used to identify and isolate the plant photoreceptor phytochrome, which
plays a major role in photoperiodic regulation of flowering in higher plants.
different
wavelengths. First, the given plant material is exposed to different doses
of light at a given wavelength and a dose response curve for the photoresponse
is plotted. Similar dose responses curves are then obtained for the other
wavelengths too, by increasing wavelength interval by 10 or 20 nm. Second,
using these dose response curves, the number of photons required to elicit
a fixed degree of the photoresponse at different wavelengths is determined.
It is naturally expected the least amount of quanta would be required for
eliciting the photoresponse at the most effective wavelength. The reciprocal
of quanta required to elicit the response are then plotted against the
wavelength and called as action spectra. The action spectra of a given
photoresponse are then compared with the absorption spectra of known photochromic
molecules. It is implicit in these comparative studies that the action
spectra so obtained closely represent the absorption spectra of photoreceptor
causing the photoresponse. In past the action spectroscopy has been successfully
used to identify and isolate the plant photoreceptor phytochrome, which
plays a major role in photoperiodic regulation of flowering in higher plants.
 the
similarity between action spectra of phototropism and absorption spectra,
it has been predicted that a carotenoid, or a flavin, or a pterin act as
putative chromophore for the photoreceptor. Arguments and evidences for
and against each of these molecules have been advanced in the past. In
recent years most people have favored flavin and pterin as potential candidates
for the chromophore, however, still few people support carotenoid as chromophore
molecule. A great deal of the controversy regarding the molecular nature
of the chromophore has been generated due to lack of identification of
the photoreceptor molecules per se. However, in recent years the remarkable
progress has been made for identification for at least few likely photoreceptor
candidates, which is described below.
the
similarity between action spectra of phototropism and absorption spectra,
it has been predicted that a carotenoid, or a flavin, or a pterin act as
putative chromophore for the photoreceptor. Arguments and evidences for
and against each of these molecules have been advanced in the past. In
recent years most people have favored flavin and pterin as potential candidates
for the chromophore, however, still few people support carotenoid as chromophore
molecule. A great deal of the controversy regarding the molecular nature
of the chromophore has been generated due to lack of identification of
the photoreceptor molecules per se. However, in recent years the remarkable
progress has been made for identification for at least few likely photoreceptor
candidates, which is described below.
 for
the phototropic curvature has added to the complexities of the identification
of the photoreceptors. The views for and against the multiple photoreceptors
have been advanced on the basis of the physiological consideration. The
recent results on identification of the photoreceptors have not still clearly
resolved the phenomena. The evidence is still incomplete to say firmly
if one or more than one photoreceptor sense phototropic blue light.
for
the phototropic curvature has added to the complexities of the identification
of the photoreceptors. The views for and against the multiple photoreceptors
have been advanced on the basis of the physiological consideration. The
recent results on identification of the photoreceptors have not still clearly
resolved the phenomena. The evidence is still incomplete to say firmly
if one or more than one photoreceptor sense phototropic blue light.